94 patients meeting criteria for insomnia were randomly assigned into zopiclone group and alprazolam group,treated for 4 weeks.
对94例失眠症患者,随机分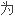
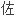
 克隆组和阿普唑仑组。疗程4周。
克隆组和阿普唑仑组。疗程4周。
94 patients meeting criteria for insomnia were randomly assigned into zopiclone group and alprazolam group,treated for 4 weeks.
对94例失眠症患者,随机分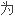
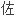
 克隆组和阿普唑仑组。疗程4周。
克隆组和阿普唑仑组。疗程4周。
A reversed phase high performance liquid chromatographic method (external standard method) was developed for the determination of zopiclone in serum.
建立了测定血清中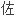
 克隆的
克隆的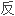 相高效液相色谱法(外标法)。
相高效液相色谱法(外标法)。
声明:以上例句、词性分类均由互联
 自动生成,部分未经过人工审核,其表达内容亦不代表本软件的观点;若发现问题,欢迎向我们指正。
自动生成,部分未经过人工审核,其表达内容亦不代表本软件的观点;若发现问题,欢迎向我们指正。
94 patients meeting criteria for insomnia were randomly assigned into zopiclone group and alprazolam group,treated for 4 weeks.
对94例失眠症患者,随机分为佐匹

 和阿普唑仑
和阿普唑仑 。疗程4周。
。疗程4周。
A reversed phase high performance liquid chromatographic method (external standard method) was developed for the determination of zopiclone in serum.
建立了测定血清中佐匹
 的
的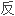 相高效液相色谱法(外标法)。
相高效液相色谱法(外标法)。
声明:以上例句、词性分类均
 网资源自动生成,部分未经过人工审核,其表达内容亦不代表本软件的观点;若发现问题,欢迎向我们指正。
网资源自动生成,部分未经过人工审核,其表达内容亦不代表本软件的观点;若发现问题,欢迎向我们指正。
94 patients meeting criteria for insomnia were randomly assigned into zopiclone group and alprazolam group,treated for 4 weeks.
对94例失眠症患 ,
,
 分为佐匹克隆组和阿普唑仑组。疗程4周。
分为佐匹克隆组和阿普唑仑组。疗程4周。
A reversed phase high performance liquid chromatographic method (external standard method) was developed for the determination of zopiclone in serum.
建立了测定血清中佐匹克隆的 相高效液相色谱法(外标法)。
相高效液相色谱法(外标法)。
声明:以上例句、词性分类均由互联网资源自
 ,部分未经过人工审核,其表达内容亦不代表本软件的观点;若发现问题,欢迎向我们指正。
,部分未经过人工审核,其表达内容亦不代表本软件的观点;若发现问题,欢迎向我们指正。
94 patients meeting criteria for insomnia were randomly assigned into zopiclone group and alprazolam group,treated for 4 weeks.
对94例失眠症患者,随机分为佐

 组和阿普唑仑组。疗程4周。
组和阿普唑仑组。疗程4周。
A reversed phase high performance liquid chromatographic method (external standard method) was developed for the determination of zopiclone in serum.
建立了测定血清中佐

 的
的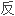 相
相
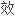
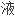 相色谱法(外标法)。
相色谱法(外标法)。
声明:以上例句、词性分类均由互联网资源自动生成,部分未经过人工审核,其表达内容亦不代表本软件的观点;若发现问题,欢迎向我们指正。
94 patients meeting criteria for insomnia were randomly assigned into zopiclone group and alprazolam group,treated for 4 weeks.
对94例失眠症患者,随机分为佐匹克隆组和阿普唑仑组。疗程4周。
A reversed phase high performance liquid chromatographic method (external standard method) was developed for the determination of zopiclone in serum.
建立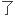

 血清中佐匹克隆的
血清中佐匹克隆的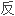 相高效液相色谱法(
相高效液相色谱法(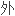
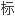 法)。
法)。
声明:以上例句、词性分类均由互联网资源自动生成,部分未经过人工审核,其表达内容亦不代表本软件的观点;若发现问题,欢迎向我们指正。
94 patients meeting criteria for insomnia were randomly assigned into zopiclone group and alprazolam group,treated for 4 weeks.
对94例失眠症患者,随机分为佐匹克隆组和阿普唑仑组。疗程4周。
A reversed phase high performance liquid chromatographic method (external standard method) was developed for the determination of zopiclone in serum.
建立了

 清中佐匹克隆的
清中佐匹克隆的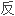 相高效液相色谱法(
相高效液相色谱法(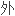
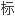 法)。
法)。
声明:以上例句、词性分类均由互联网资源自动生成,部分未经过人工审核,其表达内容亦不代表本软件的观点;若发现问题,欢迎向我们指正。
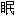
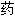 )
)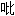 嗪哌酯
嗪哌酯94 patients meeting criteria for insomnia were randomly assigned into zopiclone group and alprazolam group,treated for 4 weeks.
对94例失 症患者,随机分为佐匹克隆组和阿普唑仑组。疗程4周。
症患者,随机分为佐匹克隆组和阿普唑仑组。疗程4周。
A reversed phase high performance liquid chromatographic method (external standard method) was developed for the determination of zopiclone in serum.
建立了测定血清中佐匹克隆的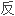 相高效液相色谱法(外标法)。
相高效液相色谱法(外标法)。
声明:以上例句、词性分类均由互联网资源自动生成,部分未经过人工审核,其表达内容
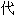 表本软件的观点;若发现问题,欢迎向我们指正。
表本软件的观点;若发现问题,欢迎向我们指正。

94 patients meeting criteria for insomnia were randomly assigned into zopiclone group and alprazolam group,treated for 4 weeks.
对94例失眠症患者,随机分为佐匹克隆组和阿普唑仑组。疗程4周。
A reversed phase high performance liquid chromatographic method (external standard method) was developed for the determination of zopiclone in serum.
建立了测定血清中佐匹克隆的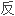 相高效液相色谱法(外标法)。
相高效液相色谱法(外标法)。
声明:以上例句、词性分类均由互联网资源自动生成,部分未经过人工审核,
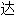 内容亦不代
内容亦不代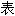 本软件的观点;若发现问题,欢迎向我们指正。
本软件的观点;若发现问题,欢迎向我们指正。
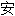
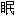
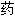 )吡嗪哌酯
)吡嗪哌酯94 patients meeting criteria for insomnia were randomly assigned into zopiclone group and alprazolam group,treated for 4 weeks.
对94例失 症患者,随机分为佐匹克隆组和阿普唑仑组。疗程4周。
症患者,随机分为佐匹克隆组和阿普唑仑组。疗程4周。
A reversed phase high performance liquid chromatographic method (external standard method) was developed for the determination of zopiclone in serum.
建立了测定血清中佐匹克隆的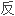 相高效液相色谱法(外标法)。
相高效液相色谱法(外标法)。
声明:以上例句、词性分类均由互联网资源自动生成,部分未经过人工审核,其 达内容亦
达内容亦
 本软件的观点;若发现问题,欢迎向我们指正。
本软件的观点;若发现问题,欢迎向我们指正。