After reiewing the issues raised in the petition, FDA determined that its standards for approal of the generic drug application for bupropion are appropriate.
FDA审查请愿书中的问题后
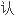 ,
, 批准盐
批准盐 安非他
安非他
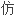
 药申请的标准是恰当的。
药申请的标准是恰当的。
After reiewing the issues raised in the petition, FDA determined that its standards for approal of the generic drug application for bupropion are appropriate.
FDA审查请愿书中的问题后
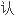 ,
, 批准盐
批准盐 安非他
安非他
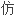
 药申请的标准是恰当的。
药申请的标准是恰当的。
声明:以上例句、词性分类均由互联网资源自动生成,部分未经过人工审核, 表达内容亦不代表本软件的观点;若发现问题,欢迎向我们指正。
表达内容亦不代表本软件的观点;若发现问题,欢迎向我们指正。
After reiewing the issues raised in the petition, FDA determined that its standards for approal of the generic drug application for bupropion are appropriate.
FDA审查请愿书中的问题后确认,其批准盐 安非他酮仿制药申请的标准是恰当的。
安非他酮仿制药申请的标准是恰当的。
声明:以上例句、词
 类均由互联网资源自动生成,部
类均由互联网资源自动生成,部 未经过人工审核,其表达内容亦不代表本软件的观点;若发现问题,欢迎向我们指正。
未经过人工审核,其表达内容亦不代表本软件的观点;若发现问题,欢迎向我们指正。
After reiewing the issues raised in the petition, FDA determined that its standards for approal of the generic drug application for bupropion are appropriate.
FDA审查请愿书中的问题后确认,其批准盐 安非他酮仿制药申请的标准是恰当的。
安非他酮仿制药申请的标准是恰当的。
声明:以上例句、词性分类均由互联网资源自动生成,部分未经过人工审核,其
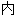 容亦不代
容亦不代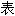 本软件的观点;若发现问题,欢迎向我们指正。
本软件的观点;若发现问题,欢迎向我们指正。
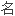
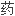 ;通用
;通用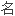
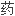 ;
; 厂
厂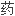
After reiewing the issues raised in the petition, FDA determined that its standards for approal of the generic drug application for bupropion are appropriate.
FDA审查请愿书中的问题后确认,其批准盐 安非他酮仿制
安非他酮仿制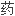 申请的标准是恰当的。
申请的标准是恰当的。
声明:以上例句、词性 类均由互联网资源自动生
类均由互联网资源自动生 ,
,
 未经过人工审核,其表达内容亦不代表本软件的观点;若发现问题,欢迎向我们指正。
未经过人工审核,其表达内容亦不代表本软件的观点;若发现问题,欢迎向我们指正。
After reiewing the issues raised in the petition, FDA determined that its standards for approal of the generic drug application for bupropion are appropriate.
FDA

 愿书中
愿书中 问题后确认,其批准盐
问题后确认,其批准盐 安非他酮仿制药申
安非他酮仿制药申
 标准是
标准是

 。
。
声明:以上例句、词性分类均由互联网资源自动生成,部分未经过人工 核,其表达内容亦不代表本软件
核,其表达内容亦不代表本软件 观点;若发现问题,欢迎向我们指正。
观点;若发现问题,欢迎向我们指正。
After reiewing the issues raised in the petition, FDA determined that its standards for approal of the generic drug application for bupropion are appropriate.
FDA 查请愿书中
查请愿书中 问题后确认,其批准盐
问题后确认,其批准盐 安非他酮仿制药申请
安非他酮仿制药申请 标准是恰当
标准是恰当 。
。

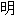 :以上例
:以上例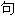 、词性分类均由互联网资源自动生成,部分未经过人工
、词性分类均由互联网资源自动生成,部分未经过人工 核,其表达内容亦不代表本软件
核,其表达内容亦不代表本软件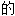 观点;若发现问题,欢迎向我们指正。
观点;若发现问题,欢迎向我们指正。
After reiewing the issues raised in the petition, FDA determined that its standards for approal of the generic drug application for bupropion are appropriate.
FDA审查请愿书中的问题后确认,其批准盐 安非他酮仿制药申请的标准是恰当的。
安非他酮仿制药申请的标准是恰当的。
声明: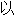
 句、词性分类均由互联网资源自动生成,部分未经过人工审核,其表达内容亦不代表本软件的观点;若发现问题,欢迎向我们指正。
句、词性分类均由互联网资源自动生成,部分未经过人工审核,其表达内容亦不代表本软件的观点;若发现问题,欢迎向我们指正。
After reiewing the issues raised in the petition, FDA determined that its standards for approal of the generic drug application for bupropion are appropriate.
FDA审查请愿书中的问题后确认,其批准盐 安非他酮仿制药申请的标准是恰当的。
安非他酮仿制药申请的标准是恰当的。
声明:以上例句、词性分类均由互联网资源自动生成,部分未经过人工审核,其
 容亦不代
容亦不代 本软件的观点;若发现问题,欢迎向我们指正。
本软件的观点;若发现问题,欢迎向我们指正。
 ;
; 用
用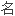
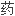 ;副厂
;副厂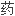
After reiewing the issues raised in the petition, FDA determined that its standards for approal of the generic drug application for bupropion are appropriate.
FDA审查请愿书中的问题后确认,其批准盐 安非他酮仿制
安非他酮仿制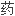 申请的标准是恰当的。
申请的标准是恰当的。
声明:以上例句、词性分类均由互联网资源自动生成,部分未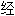
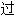
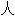 工审核,其表达内容亦不代表本软件的观点;若发现问题,欢迎向我们指正。
工审核,其表达内容亦不代表本软件的观点;若发现问题,欢迎向我们指正。